Research highlights
 Revealing the colour of cancer
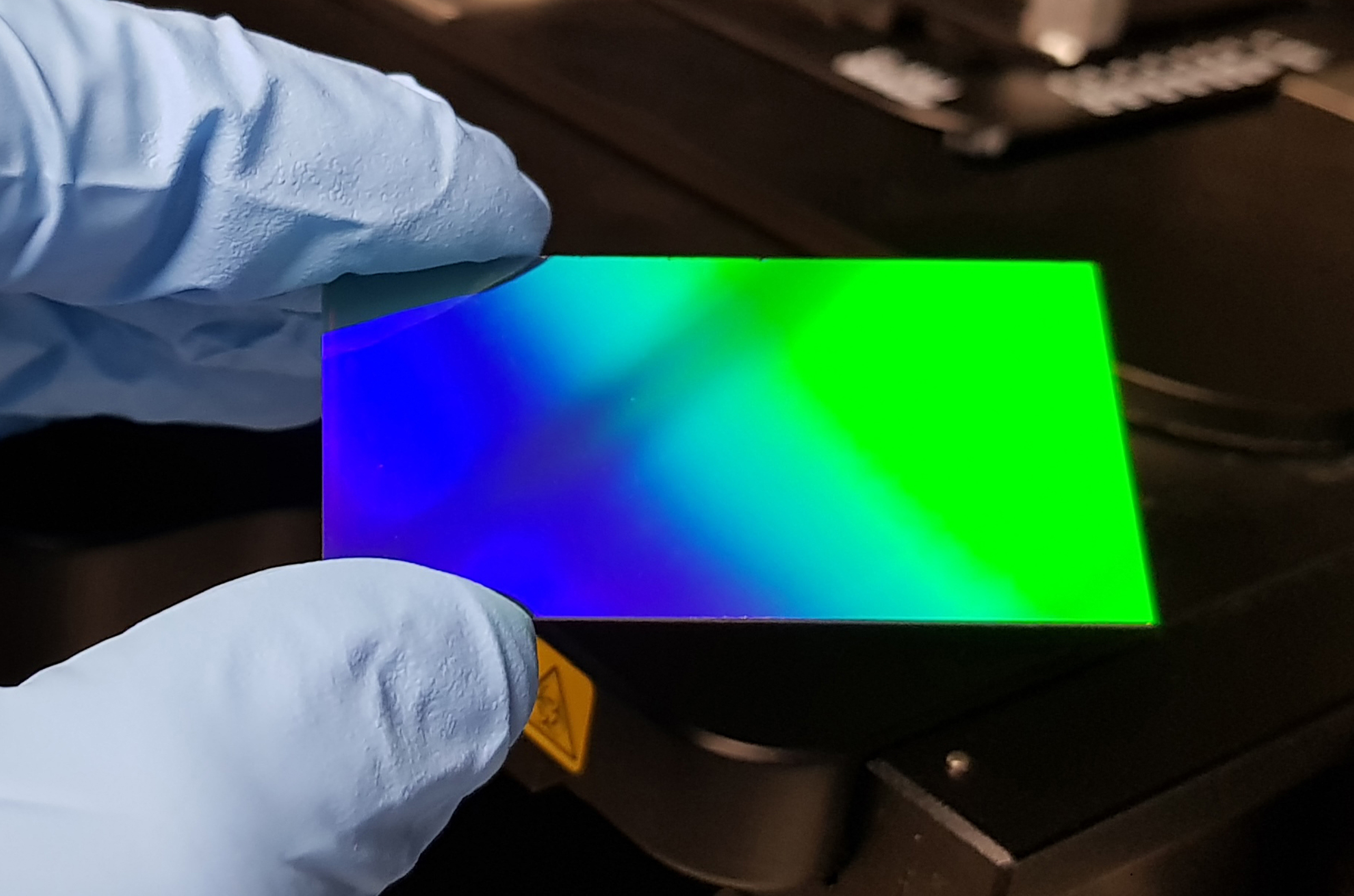
Revealing the colour of cancer
An innovative microscope slide, NanoMslide, developed at La Trobe University is promising to revolutionise medical imaging after researchers demonstrated that it can be used to detect breast cancer cells in patients. A study published today in Nature demonstrates that by modifying the surface of conventional microscope slides at the nanoscale, biological structures and cells take on a striking colour contrast which can be used to instantly detect disease. Project lead, Professor Brian Abbey has spent the past five years developing the technology at La Trobe University with co-inventor Dr Eugeniu Balaur.
Cancer causing genes
Cancer is caused by mutations affecting cancer-causing genes. Discovering the mutated genes that drive tumour growth and metastasis is the first step towards finding new ways to diagnose and treat cancers. Dr Andrew Farrell, and LIMS Group Leaders, Dr Helena Richardson and Professor Patrick Humbert (together with collaborators from IMBA Austria and UBC Canada) have discovered a new cancer-causing gene, TSPAN6. They found that TSPAN6 has a tumour suppressor function in cancers driven by the RAS oncogene, such as lung and pancreatic cancers. Their findings, published in Oncogene (Nature), show that several cell-communication pathways are defective in TSPAN6 mutant RAS-driven cancer cells. This discovery may provide new ways of therapeutically targeting these cancers.
Can ingesting cow milk-derived vesicles improve cancer treatment outcomes?
Ground-breaking research from LIMS scientists may lead to the development of new options for cancer treatments, particularly for advanced cancer patients who have undergone surgery to remove the primary tumour. Extracellular vesicles (EVs) are tiny vesicles released by cells that contain important proteins, nucleic acids and lipids. EVs can facilitate communication between cells and play an important role in cancer development and progression. For years, there has been speculation that if EVs present in food like cow’s milk could be ingested by a cancer patient, then the EVs could deliver important “cargo” to the body’s organs and regulate cancer progression. Until now this remained unproven. Research published in Nature Communications, from Professor Suresh Mathivanan’s team at the La Trobe Institute for Molecular Science (LIMS), shows it is indeed possible.
Visualising DNA damage and repair
Researchers have mapped, for the first time, some of the specific molecular interactions that repair DNA double strand breaks that cause cancer, genetic abnormalities, and other types of neurodegeneration and immune disease. The team, led by Dr Donna Whelan, used advanced microscopy techniques to visualise the first responding proteins at the damaged site and their critical role in the repair process. By applying cutting-edge, single-molecule, super-resolution techniques, the team were able to identify how individual helper proteins interact with each other, and with the DNA itself, to repair breaks. The research, published in the prestigious journal Proceedings of the National Academy of Sciences of the United States of America, offers new insight into the optimal pathway for DNA repair in healthy cells.
